Urinary Tract Infections (UTIs) are the most commonly acquired infection in a hospital setting and are often the result of indwelling catheters. Catheter Associated Urinary Tract Infections (CAUTIs) result in an estimated 13,000 deaths annually, as well as an increased length of stay by 2-4 days and $400-500 million in additional costs per year nationally. One effective measure to reduce CAUTIs is to decrease the number of days a patient has an indwelling catheter. External urine collection devices have proven to be an effective alternative to indwelling catheters. These devices are currently available for males (e.g. ReliafitTM, condom catheters, etc.); however, the industry falls behind in providing an effective solution for females.
The uCol device was developed as a solution to reduce or eliminate the use of an indwelling catheter for the female patient population and provide an effective urinary collection device for both continent and incontinent women for a variety of indications, including bed rest, wound healing, and end-of-life care.
The idea for the uCol device conceptualized by Mary Tibbe, MSN, RN, CCRN, AGCNS-BC, clinical nurse specialist in the Cardiothoracic Critical Care Unit (CTCCU) at Butterworth Hospital. Tibbe works in the critical care units to improve patient care and related outcomes, including preventing healthcare associated issues such as CAUTIs. Tibbe was frustrated that there was no urine collection device as an alternative to indwelling catheters for females. For males, a condom catheter could be used in lieu of a Foley catheter in some situations, but no similar option was available for women. This often results in women requiring longer time with an indwelling catheter as no external option is available.

Tibbe presented the problem to Spectrum Health Innovations. Spectrum Health Innovations (SHI) is an entity within the Spectrum Health system that focuses on the development and launch of promising new health care products and technologies, such as medical devices, surgical tools, nursing products, hospital equipment, therapy equipment and software. SHI aims to develop products and technologies that can improve the quality of care at Spectrum Health, many of which may also have broader commercial potential and applicability to health care systems beyond Spectrum Health.
From fall 2015 through 2017, Spectrum Health partnered with the Applied Medical Device Institute through Grand Valley State University to develop the device. External funding was obtained through a MI-Kickstart grant to refine and test the product. This work has resulted in submission of a provisional patent.
Mary Tibbe remained integrally involved to inform this process. Tibbe was consulted for the original prototype and all design changes. The prototypes of the device were tested on volunteers, including Tibbe and several nurses in the organization.
In February 2018, Spectrum Health senior nurse researcher Dr. Jennifer Kaiser, PhD, MSN, RN, in collaboration with SHI, was awarded an $88,000 grant from the Michigan Translational Research and Commercialization (MTRAC) Innovation Hub for Advanced Transportation to launch a clinical trial of the uCol device. This is the first Food and Drug Administration (FDA)-regulated nursing clinical trial conducted at Spectrum Health, with a nurse as the primary investigator.
The trial involves testing of the device in patients. For the indication of incontinence management, 64 residents and subacute rehab patients at the Spectrum Health Fuller Continuing Care campus will wear the device for up to 24 hours. Comparatively, 64 cardiothoracic surgical patients will trial the device in lieu of a Foley catheter placed intraoperatively.
The trial requires significant support and involvement of nursing staff across Spectrum Health. Most clinical trials of devices are conducted to minimize any request for care from nursing outside of standard practice. For the uCol device trial, clinical nurses will actually be the ones who apply, maintain, and evaluate the device. All of the trial data will be generated from nursing staff. From March 2018 through May 2018, 89 nursing staff from continuing care, surgical services, the Cardiovascular Recovery Unit (CVRU), and the 7MHC unit were trained to apply and manage the device. The first devices were applied to patients in June 2018, and the trial is projected to continue until February 2019. This is truly a nursing clinical trial, from idea conception through all aspects of the research.
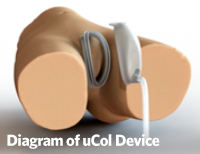 The uCol device is a completely external urinary collection device. It is a two-piece design with a ring and cup that interlock but can be separated for cleaning and perineal care. The ring adheres to the patient via a skin-friendly medical adhesive used in wound care products. The cup can connect to a port and tubing that can lead to a Foley collection bag or leg bag. Suction may be applied if necessary. The cup may also be used without the tubing and voided into if necessary. For continent patients, the ring can be worn without the cup until voiding. This unique design with multiple options for application expands the usability and comfort of the device.
The uCol device is a completely external urinary collection device. It is a two-piece design with a ring and cup that interlock but can be separated for cleaning and perineal care. The ring adheres to the patient via a skin-friendly medical adhesive used in wound care products. The cup can connect to a port and tubing that can lead to a Foley collection bag or leg bag. Suction may be applied if necessary. The cup may also be used without the tubing and voided into if necessary. For continent patients, the ring can be worn without the cup until voiding. This unique design with multiple options for application expands the usability and comfort of the device.
At the conclusion of the trial, 128 patients will use and assess the device. Endpoints include device efficacy, patient comfort, and staff satisfaction. The ultimate outcome of the trial is to establish an effective alternative to indwelling catheters for female patients in some clinical situations and to reduce the time that indwelling catheters are used. This will reduce the development of CAUTIs in this population. The second objective is to provide an effective device for the collection of urine for both continent and incontinent females for a variety of indications. The creation and production of the uCol device will provide female patients a non-invasive, effective solution for urinary management in a variety of settings.
uCol Team
Jennifer Kaiser, PhD, MSN, RN, senior nurse researcher, nursing practice and development
Mary Tibbe, MSN, RN, CCRN AGCNS-BC clinical nurse specialist, cardiovascular services
Kris Emery, BSN, RN, CHIE, clinical innovation nurse, Spectrum Health Innovations
Eric Van Middendorp, MSE, biomedical engineer, Spectrum Health Innovations
Andy Heuerman, MET, BS, CHIE, product development specialist, Spectrum Health Innovations
